There is a line of thinking, you may have encountered it, that says something to the effect of "there is no such thing as consensus in science." The argument goes that in science we never prove, only disprove and we are always discovering, learning, tweaking and we should always be holding what we believe to be true, especially the theories and laws most dear to us, with skepticism. Therefore, we should not or cannot ever have consensus. There should always be room for debate in science and consensus is a tool of the powers of authority to stop those that would challenge this authority.
The real difficulty with this line of argument is that it takes something that is true and good about science (that we only disprove, that we constantly amend our theories when new observations or information is collected, that we should remain skeptical) and takes its logical end creating such epistemological relativism that would dictate we can never know anything or ever do anything with that knowledge. Which is demonstrably not the case. We do know things and we do things with this knowledge.
Let's think about how this applies to engineering. We've studied steel. Steel is great; it's fairly plentiful (rather it's components are), it's very strong, it's relatively easy if somewhat energy intensive to make stuff with. So we have an idea: let's build stuff with it! Let's use it to build some buildings! Modest at first but eventually, maybe we can build some really tall buildings! Now if your position is really that we cannot build scientific consensus you'll say: "No, no! We need to continue to learn about steel! Perhaps there is some hidden property that we're unaware of that would make it unsafe to build with! What if there is an as-yet-undiscovered reaction that occurs when it reaches a certain elevation that makes it impossible to build tall buildings with?" But we do build with steel. We've reached a level of confidence that allows us to say "we know enough about this material, we understand its properties, let's move on and do some engineering with it."
While it is true that nothing in science is ever proven in the strictest sense, confidence is a technical term for how statistically likely it is for future experiments or observations to hold with past experiments and observations. So while we might not have proven that steel is a good building material, the statistics are very much in our favor.
We also do have what we call scientific facts. A fact is a piece of knowledge that, again, has been tested over and over and over to such an extent that statistically we have very strong confidence that it is for practical purposes "true." One of the go to examples of this would be if you hold an object in your hand (that has greater density than air) and let go of it, it will fall. Steel's high strength is a fact. Atoms consist of protons, neutrons and electrons: fact. Now could we discover new aspects of the natural world that counter these observations? We could. It's just very, very unlikely.
"Ah!" Says the anti-consensus line of thinking. "But in science there is no "truth." We've been wrong before and we could be wrong now!" Yes, many many scientific facts, laws and theories that were consensus for very long periods of time have not held up to the scrutiny of the scientific method and new evidence. It's quite true. We once believed in something called "the aether," a substance through which light waves could propagate. It doesn't exist; we now know that light and other electromagnetic waves are self-propagating. We once believed there was a substance called "phlogiston" within all combustible materials. We had lots of evidence based on observation and experiment that such a thing existed. It turns out we were wrong.
Scientists and consensus can get locked into what is "accepted" and fail to pick up on evidence that would lead us to new theories, facts and laws that better explain our observations. This is a very real aspect of the scientific method. But saying that we cannot ever have enough confidence in our observations to do anything with them is throwing the baby out with the bath water. And, again, demonstrably false. Engineering is nothing but taking scientific facts, measurements, theories, etc and applying them. We reach consensus whenever we build new infrastructure and technology. Whenever we develop medicine (and yes, sometimes we make mistakes) we are reaching consensus. When I know that jumping from a great height is a bad idea because gravity will accelerate me quickly to the ground and severely injure or kill me, I'm working with scientific consensus.
You may see this argument applied to climate science and climate change. That because science never proves anything, saying that there is a consensus is antithetical to the scientific method and merely serves to silence dissenters. It prevents us from making new discoveries. But here's what we know: it is a scientific fact (in the technical sense) that carbon dioxide and other gases trap heat in our atmosphere. It is a historical and scientific fact that humans have added a bunch of rampant carbon dioxide to Earth's atmosphere. Therefore, we can infer (and have measured) a rise in average atmospheric and sea surface temperatures. We have observed ice that has existed since humans have melting. We have a very, very high level of confidence in these things. And we have a very high level of confidence that Earth's atmosphere will continue to warm and that this will have other effects on our climate.
Do we know exactly what will happen with the climate? No, of course not. We have some decent models but there are a host of variables we can't predict very well (mainly the social variables, i.e. what are humans and governments going to do as we move into the coming decades). Humans are notoriously difficult to predict. But just because we don't have a perfect climate model with extremely high confidence doesn't mean we don't still have some basic facts that allow us to act in a logical, reasonable way.
Good scientists are always skeptical, always open-minded. Good scientists do their best to truly think critically and examine if their observations are in conflict with what they believe. But good science also allows us to create, to heal, to feed, to protect. Good science allows us to act and not only study.
Sources:
Kuhn, Thomas. The Structure of Scientific Revolutions. Chicago and London: The University of Chicago Press, 1962.
https://www.visionlearning.com/en/library/Process-of-Science/49/Uncertainty-Error-and-Confidence/157
https://eic.rsc.org/feature/the-logic-of-phlogiston/2000126.article
https://ncse.com/library-resource/definitions-fact-theory-law-scientific-work
Wednesday, December 12, 2018
Saturday, August 11, 2018
The Speed of Light and Time Dilation
So light is fast. But
its speed isn’t necessarily special. It
just travels at the highest speed the universe allows. Photons (the particles of light) have no mass
so, like all things without mass, they have nothing to slow them down. So off they go as fast as they can! Why is there a universal speed limit? The answer to this is, like other questions
about the fundamental nature of the universe and laws of physics, we simply
could not exist in a universe with infinite speeds. There are two things that would go wrong for
us: 1. It would take infinite energy to
give something mass and so things with mass (like us) couldn’t exist. 2. Time (or to be more precise, really,
space-time) wouldn’t exist. Light
travels at the fastest speed a signal can be sent in our universe. Because we have a finite signal speed it
means that events have distance and time between them. This is quite odd to say, but an infinite
speed of light or infinite signal speed would mean that there are not distances
and times between events. How would a
particle with infinite speed actually behave?
A signal (or particle) with infinite speed would exist everywhere in the
universe at once. These particles would
never take any amount of time to go anywhere and would never need to travel
anywhere because they all would already be everywhere forever. So while such a universe could exist we
wouldn’t be around to observe it.
Nothing, really, would be around.
Because the speed of light is always at the fastest possible
speed the universe allows this has a weird byproduct: light’s speed is not
relative to the observer. It is always
the same. Here’s a little more detail
about what that means and following, a little about why that’s weird. Imagine someone on a train car going 100 km/h,
you’re standing still watching the train car go by. Now imagine they throw a ball at 50 km/h. From their perspective the ball is going 50
km/h. But from your perspective the
train and the ball add their speeds together and the ball is going 150 km/h. For most moving objects in the universe you
have to measure their speed from a particular frame of reference. That is, are we measuring the ball’s speed
from your frame or from the person who is throwing the ball? We will get different answers depending on
which frame we decide to measure from.
But because light always goes at the maximum speed allowed by the
universe, this does not happen for light.
Let’s go back to the train car only this time the person on board has a
flashlight. They’re traveling along
again at 100 km/h. They turn on the
flashlight. From their perspective the
photons exiting the flashlight are going c (the speed of light, about 300,000
km/s). From your perspective the photons are traveling? Also c, exactly the same speed. Not c + 100 km/h.
Here’s the weird thing…at least weird from our day to day
experience: the fact that light travels
at the universal speed limit and its speed doesn’t change based on any frames
of reference leads to time being experienced differently when travelling at
different speeds. Let’s go back to the
train car. This time, imagine there are
mirrors inside the car and a beam of light bouncing between them. The light beam is bouncing straight back and
forth and takes some very small amount of time to make one bounce. This is what it might look like, the back
bars being the mirrors and the blue bar being the light beam:
Now, let’s set the train car moving. Now the light beam must bounce at a diagonal
and travel a slightly longer distance in order to “catch up” with the moving
car. Here’s what this looks like, again,
the black bars are the mirrors, spaced out to show the movement of the train
car:
Just like with the ball thrower, with most objects the train
will just impart its speed on the object and we will see it go faster to keep
up with the moving train across the slightly longer diagonals. But here’s where things get weird. Remember that light cannot go any faster and
that it always goes at the speed c regardless of frame of reference (so its
speed is the same if you are observing from outside the train or on the
car). If the speed can’t increase it
seems we may be stuck with a problem: the light has to travel a longer distance
in the same amount of time but it simply can’t because the speed of light is
conserved no matter what. Here’s the
punchline: instead of adjusting the speed of light, the universe adjusts how
much time passes for each frame of reference.
From outside the train time on the train actually slows down so the
light has enough time to get back and forth from mirror to mirror. From inside the train time appears to move at
a normal rate. But from outside, time is
moving slower.
This is the phenomenon known as time dilation. We don’t really start to see significant
effects until we get pretty close to the speed of light but this happens every
time you move with respect to someone/something else. Every time you get in a car, train, plane,
skateboard, bicycle, heck even walking, time slows down for you just a little
bit. So be wise, friends. Choose something productive to do with all
your extra nanoseconds.
Some references:
Some references:
Cox, Brian and Jeff Forshaw.
Why Does E=MC2?
Boston: Da Capo Press, 2009.
Saturday, March 17, 2018
What is a Chemical Element?
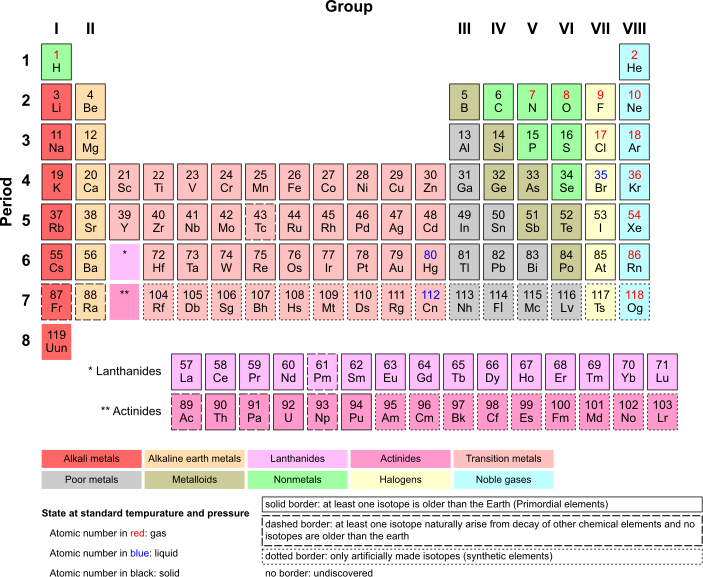
Source: Wikimedia Commons
The periodic table of the elements organizes all the chemical elements. Elements are, in most ways, the fundamental building blocks of the kind of matter that we are most familiar with. You can go smaller than an element and find protons, neutrons, electrons and quarks (and possibly go smaller/more fundamental and find strings). You can go bigger as well and find molecules built of several elements. Most of the matter we interact with is made up of molecules containing multiple elements. But in order to have water or wood or a zebra you need atoms of various chemical elements.
While most of the stuff around us on Earth is made of compounds, often quite complex compounds when it comes to what makes up living things, we do have some experience with stuff made from single elements. This is known as a "natural state." Graphite, the stuff we use to make pencils write, is pure carbon arranged in layers that can easily slip off the tip of the pencil and onto the paper. Diamond is actually one giant carbon molecule. Oxygen, while we have a less tangible relationship with it, is perhaps the most important substance to us for, y'know, not dying.
We also have experience with some metals as single element substances. Aluminum is used to make foil and cans, silver has been used to make well...silverware and other household items, gold is used in jewelry and wires. While these metals are often found naturally occurring in a larger compounds we have figured out how to use chemistry to remove the atoms of just the metal. Natural state metals are extremely useful because they tend to be extremely malleable (you can mash them into a shape, like clay...though, this may require some pretty extreme heat) and conductive (these properties are why gold is so useful for wires).
Though it may be a bit indirect, we also may have some experience with neon in the form of neon lights or signs. We use several other gases that are chemically similar to neon to make these lights. When excited with electricity, these gases will produce different colors.
But these are all experiences of substances at a gross macro level. We can talk about malleability of metals or how neon glows when excited by electricity. The factor that makes an atom a given element, though, is the number of protons. You can change the number of neutrons (and get an isotope) or the number of electrons (and get an ion) but if you change the number of protons in an atom, you change the element. Thinking about how that translates to the macro level can seem...a little strange.
Take carbon again as an example. Right next to carbon on the periodic table is nitrogen which, among other things, makes up most of Earth's atmosphere. It seems, for lack of a better word, weird, that adding a proton to the nucleus of an atom would make carbon into a gas. But that exact thing happens all the time! Well, in geologic terms. All elements have what is called a "half-life." This is the time it takes for half of a given sample to undergo a transformation into another element. Energy is required to hold even many smaller atoms together and so they do not last forever. In the case of carbon, carbon 14 (which has 6 protons and 8 neutrons) "decays" over time and becomes nitrogen 14 (7 protons and 7 neutrons).
What we more commonly think of as "radioactive" substances are substances that do this much, much more quickly. Larger atoms like thorium, plutonium and uranium also tend to decay in a way that involves particles being shot out of their nucleus. It is these particles, along with the much faster rate (carbon 14 decays in about 6,000 years, the most stable isotope of thorium in just around 18 days) that makes these isotopes dangerous. But it also means they actually turn into other elements on the scale of human lives.
The alchemists' quest to turn lead into gold may not really have succeeded, but humans have found ways using particle accelerators to ram protons into atoms so fast that they stick, changing one element into another. While experimenters have succeeded in turning other elements, like bismuth, into gold, this is only at the level of about a thousand atoms in a given trial. This is about a billionth of a billionth of a gram of gold. So while it may not be what the alchemists were looking for, it does experimentally show that what we think of at the macro level as a fairly stable, concrete thing, is changeable and just comes down to the number of protons found in the nucleus.
So what is a chemical element? Like many things in the natural world, it depends on what scale you're asking. At the macro level gold is a shiny metal substance that's really good for making things like rings and wires and such. But at the atomic level it's just the number of protons in the nucleus. If you add a proton to gold it becomes mercury and, at least at room temperature, it melts.
Sources:
http://www.preservearticles.com/201012302013/carbon-14-deca.html
https://cosmosmagazine.com/chemistry/how-noble-gases-give-us-neon-lights
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/rocks/metalsrev1.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/add_gateway_pre_2011/chemical/nanochemistryrev1.shtml
https://www.livescience.com/23232-smallest-ingredients-universe-physics.html
https://www.youtube.com/watch?v=7g-WOMXe6Mo
https://www.scientificamerican.com/article/fact-or-fiction-lead-can-be-turned-into-gold/
Subscribe to:
Posts (Atom)

